Making clinical research crystal clear
Redaction and anonymisation services
Data privacy is important. Patients and the public are demanding more transparency in clinical research. In response, regulatory authorities, including the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), and Health Canada (HC), require clinical trial sponsors to make clinical trial documents publicly available. These documents may contain protected personal data (PPD) which is information that could be used to identify individuals. Commercially confidential information (CCI) may also be present in these documents. Clinical study sponsors must anonymise PPD and CCI to reduce the risk of participant identification and protect CCI. They must achieve this without reducing the scientific utility or value of the documents.
Krystelis Services in Support of EU CTR 536/2014
Krystelis Services in support of EMA Policy 0070 and Health Canada Policy on the Public Release of Clinical Information (PRCI)


We offer a full-end-to-end document redaction and anonymisation service
Assessment of source documents for PPD
Re-identification risk-assessment
Preparation of an anonymisation plan
Identification of CCI
Literature search to confirm CCI
Anonymisation/redaction of PPD
Redaction of CCI and preparation of justification table/proposed redaction control sheet
Preparation of the anonymisation report
Krystelis Subject Matter Experts (SMEs) have been working in this area since 2012. During this time, they have supported redaction and anonymisation activities for many clinical trial sponsors, from small biotechs to top-10 pharmaceutical companies (Figure below):
Benefits of working with Krystelis
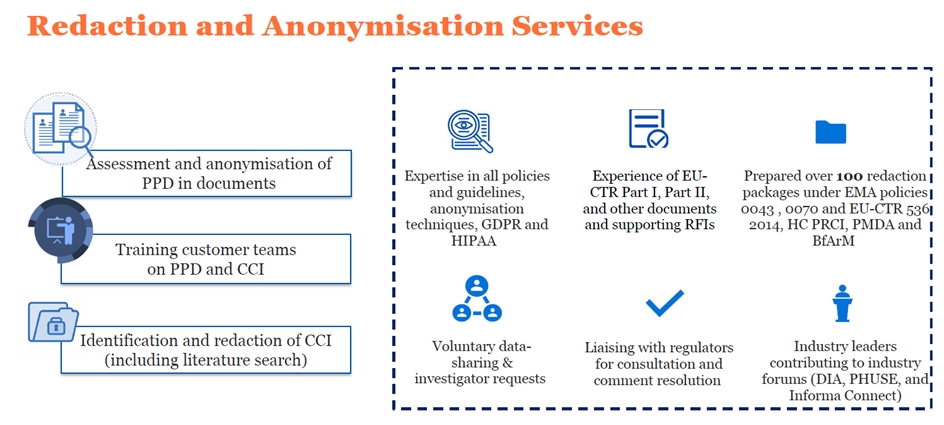
Deep expertise in all policies and guidelines, anonymisation techniques, GDPR and HIPAA
Our experience of voluntary data-sharing requests, investigator requests, and regulatory obligations
Our experience in gap analysis and preparing redacted documents in compliance with EU CTR
We have prepared redacted packages under EMA Policies 0043 and 0070, HC PRCI, PMDA and BfArM
Our experience liaising with regulators for consultation and comment resolution
Insights gained from our contributions to industry forums (DIA, PHUSE, and Informa Connect)