Making clinical research crystal clear
Plain language services
There has never been more demand for effective plain language communication.
Delivering breakthrough treatments involves many stakeholders – including researchers, regulators, grant approvers, healthcare professionals and patients. Everyone involved benefits from effective communication, however, the industry has only recently considered how best to communicate clinical research information more comprehensively to patients and the general public. This is recognised by global regulators and policymakers through regulatory requirements for plain language communication:
Delivering breakthrough treatments involves many stakeholders – including researchers, regulators, grant approvers, healthcare professionals and patients. Everyone involved benefits from effective communication, however, the industry has only recently considered how best to communicate clinical research information more comprehensively to patients and the general public. This is recognised by global regulators and policymakers through regulatory requirements for plain language communication:
- US FDA 21 CFR 50 Subpart B and Article 28 & 29 of European Union Clinical Trial Regulation (EU CTR) 536/2014 for the informed consent
- EU CTR 536/2014 Article 37 and proposed changes to UK’s Clinical Trial Legislation for plain language summaries (layperson summaries)
- Part 13, Chapter 1 of the UK’s Human Medicines Regulations (HMRs) and Article 54, Article 55 and Article 59 (Title V) of Directive 2001/83/EC for patient information leaflet


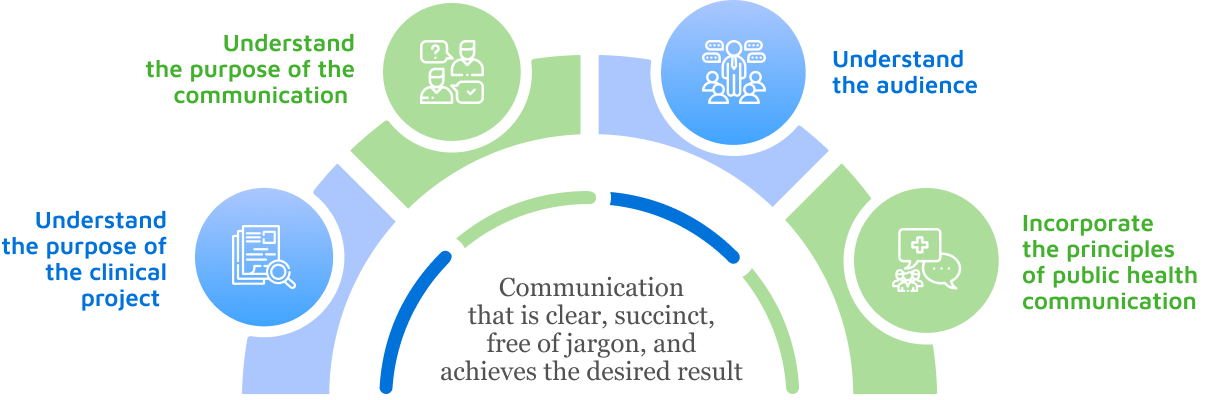
We offer world-class plain language services for clinical research by applying
Our deep understanding of the complexity of public health communication
Our extensive experience in health literacy and numeracy principles
Social listening to understand how patients and public discuss health topics
Engaging with patient partners to better understand disease areas
Experience of our experts
Completed over 150 plain language communication materials
Delivered both content and graphic design support
Implemented plain language processes for study sponsors
Trained clinical study teams on plain language concepts and processes
Published articles on key developments in the plain language domain
Contributed to the development of industry standards


Services offered
- Plain language writing services, including but not limited to following documents
Informed consent documents
(ICDs)
Trial consent element documents (TCEDs)
Plain language protocol synopses
Plain language summaries (PLSs)
for clinical trial results
Plain language summaries of publications
Patient information leaflets
Patient recruitment material
Public health education material
- Health literacy review of existing plain language material
- Review of material by a patient representative panel (patient panel review) or public representatives (non-scientific panel review)
- Facilitation of training for study teams on plain language concepts and processes
- Graphic design support for plain language material (infographic, template designing, comic and video formats)
- Advisory services, including understanding the implications of the regulations and developing process documents, e.g., SOPs, templates, glossaries, training material
- Translation and dissemination services