Making clinical research crystal clear
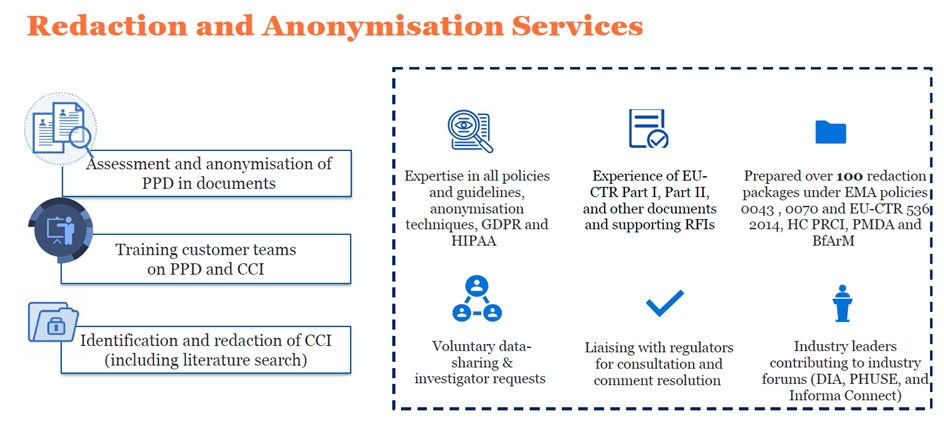
Redaction and anonymisation services
Data privacy is important. Patients and the public are demanding more transparency in clinical research. In response, regulatory authorities, including the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), and Health Canada (HC), require clinical trial sponsors to make clinical trial documents publicly available. These documents may contain protected personal data (PPD), which is information that could be used to identify individuals. Commercially confidential information (CCI) may also be present in these documents. Clinical study sponsors must anonymise PPD and CCI to reduce the risk of participant identification and protect CCI. They must achieve this without reducing the scientific utility or value of the documents.
Krystelis Services in Support of EU CTR 536/2014
Krystelis Services in support of
EMA Policy 0070 and Health Canada Policy
on the Public Release of Clinical Information (PRCI)
Redacting and/or anonymising documents to meet regulatory requirements can be time-consuming and technically challenging. Many sponsors outsource these activities to specialised vendors such as Krystelis.