Making clinical research crystal clear
Medical Writing and Quality Review Services
Document writing for regulatory and commercial purposes is a fundamental capability supporting clinical research. Scientifically accurate and high-quality documents are important to ensure clear communication of messages to diverse audiences and stakeholders. Krystelis has the necessary scientific and industry experience to deliver the full range of writing services. We can support ad hoc documents or deliver through a functional service provider engagement. Our writing team has:
- Extensive experience in providing writing services to the life sciences industry across therapeutic areas
- The capability and capacity to support you across a wide range of document types, throughout all phases of clinical development and commercialisation
- Skillsets to prepare logically organised and succinct scientific content
- Data visualisation approaches that effectively convey key messages to diverse audiences – regulatory, business, and the public, including clinical study participants
- A deep understanding of the interdependencies of disciplines (e.g., CMC, pharmacology, pharmacokinetics, clinical, regulatory) from a strategic planning perspective
- Robust project management and facilitation skills to help negotiate with internal and external stakeholders. We always deliver to deadlines

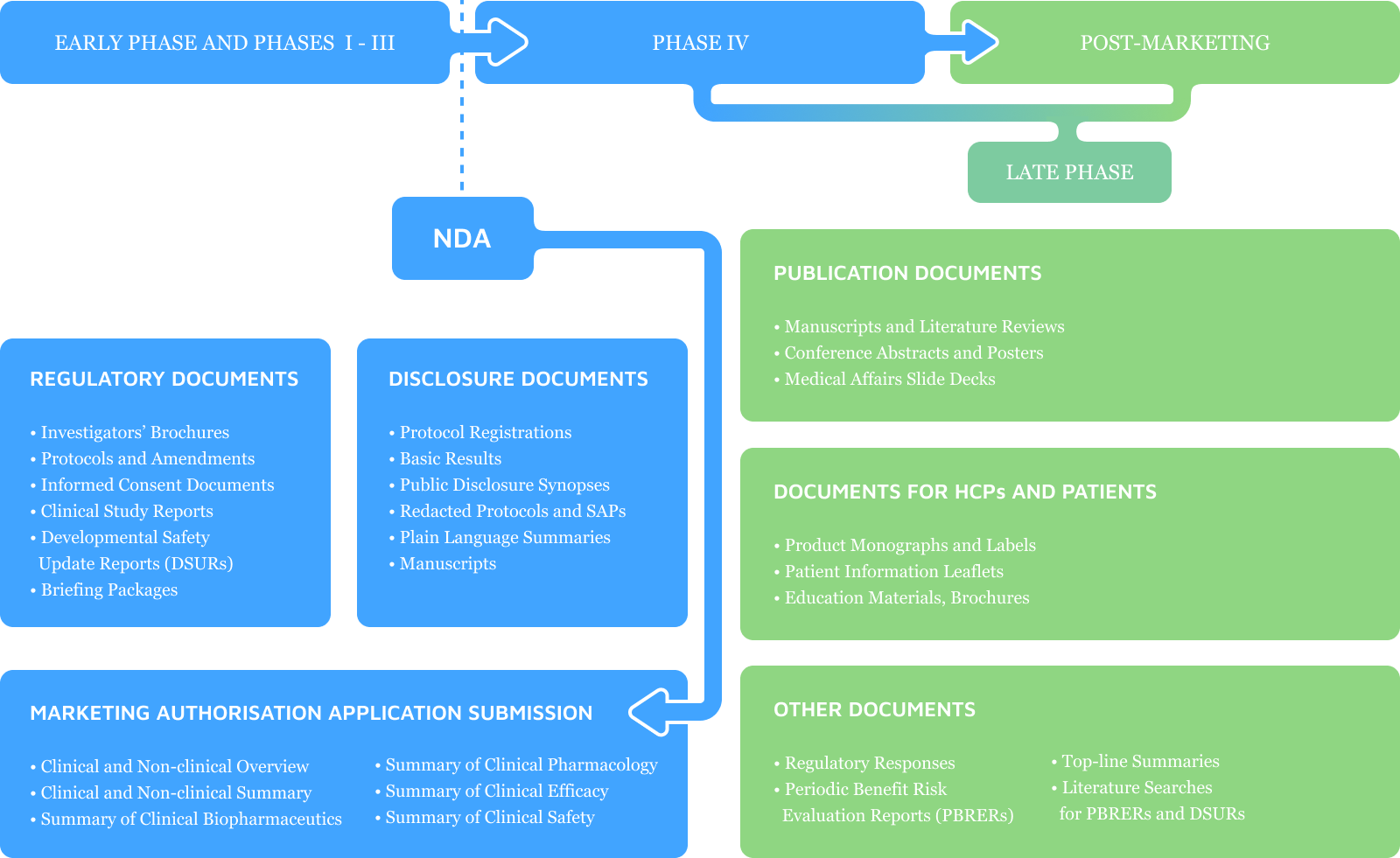
Our writing services across the
drug development process

Quality review services
Poor quality regulatory documents can impact drug development timelines and your company's reputation. Multiple reviewers, tight timelines, and changing requirements contribute to poor document quality. Document authors are typically responsible for ensuring quality. These authors are usually in high demand, are high cost, and may not have the time, experience, or skill sets required to do a thorough quality review. By using Krystelis's independent quality review services, you can increase the quality of clinical trial documents while accelerating drug approval timelines and reducing costs.

Our quality review service includes:
Benefits of independent quality
review services delivered by Krystelis
What differentiates us

